A new study from Great Britain underscores the importance of early detection of Barrett’s Esophagus in the fight to stop Esophageal Cancer before it starts. It provides new information that shows Barrett’s Esophagus starts as stomach cells, how they transform to resemble so-called “goblet cells” of the intestines and identifies the genes involved in that switch.
You can download the study here.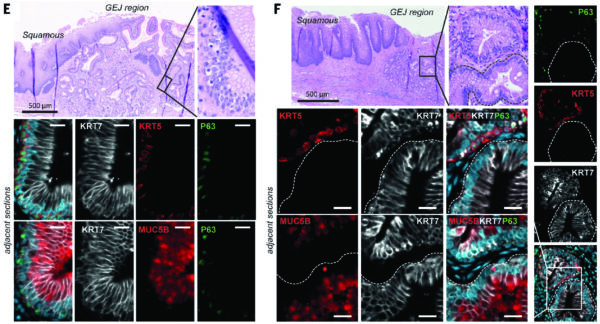
From a University of Cambridge News Release:
Abnormal cells that go on develop into Esophageal Cancer – cancer that affects the tube connecting the mouth and stomach – start life as cells of the stomach, according to scientists at the University of Cambridge.
The study, published in Science, found that a particular subtype of Esophageal Cancer known as Esophageal Adenocarcinoma is always preceded by Barrett’s Esophagus – abnormal cells of the Esophagus – even if these cells are no longer visible at the time of cancer diagnosis. This confirms that screening for Barrett’s is an important approach to Esophageal Cancer control.
Cancer of the Esophagus is the sixth most deadly cancer, and Esophageal Adenocarcinoma is on the rise in western countries. Scientists and doctors have known for some time that the development of this cancer is linked with Barrett’s Esophagus, which shows up in endoscopy as a pink ‘patch’ in the surface of the esophagus and affects around one out of every 100 to 200 people in the United Kingdom – and between 3 and 13 people out of 100 with this condition will go on to develop Esophageal Adenocarcinoma in their lifetime. However, the question of where these abnormal cells come from has been a mystery that has baffled scientists for decades.
A multidisciplinary group of scientists led by Professor Rebecca Fitzgerald at the Medical Research Council Cancer Unit, University of Cambridge, today provides the most comprehensive explanation to date.
Dr. Lizhe Zhuang, the joint first author of the study, said: “It’s intriguing that, although Barrett’s Esophagus predominately occurs in the lower part of esophagus close to the stomach, it has so-called ‘goblet cells’ resembling a much more distant organ, the small intestine. Over the past twenty years, there have been at least six different hypotheses about the origin of Barrett’s Esophagus. Using the latest techniques, we believe we have arrived at an answer to this mystery.”
The research team analyzed tissue samples from patients with Barrett’s Esophagus and from organ donors who have never had the condition. The samples were collected as part of the Cambridge Biorepository for Translational Medicine at Addenbrooke’s Hospital, part of Cambridge University Hospitals NHS Foundation Trust.
Lead authors Dr. Karol Nowicki-Osuch and Dr. Lizhe Zhuang established a detailed ‘atlas’ of human cells and tissues from all possible origins of Barrett’s Esophagus, including esophageal submucosal glands, an elusive tissue structure that acts in a similar way to saliva glands and has never before been isolated from fresh human tissue.
The researchers then compared the maps of cells from healthy tissues, Barrett’s Esophagus and Esophageal Adenocarcinoma using a number of state-of-the-art molecular technologies. These included single-cell RNA sequencing, a powerful technology that enables researchers to investigate the functions of a large number of individual cells. They also looked at methylation profiles – chemical modifications to the DNA of cells in the tissue – and at genetic linage to trace back where a particular cell type originated.
The results showed a striking similarity between stomach cells and Barrett’s Esophagus, suggesting that the cells at the very top of the stomach can be reprogrammed to adopt a new tissue identity, becoming more like intestine cells, and replace the esophageal cells. Furthermore, in this new study, the team showed that two genes, MYC and HNF4A, are the keys that switch the tissue identity from the stomach to intestinal cells.
Dr. Karol Nowicki-Osuch, the joint first author of the study, said: “The techniques we used have shown us the internal processes that happen in the stomach cells when they become Barrett’s. The big question now is: what triggers these genes? It’s likely to be a complex combination of factors that include bile acid reflux (often felt as heartburn) and other risk factors, such as obesity, age, male sex and Caucasian ethnicity.”
Importantly, the researchers found that all esophageal adenocarcinoma cells begin as stomach cells before transforming into Barrett’s cells and then into cancer cells.
Professor Fitzgerald added: “Even if the pre-cancerous Barrett’s is not visible at the time of cancer diagnosis, our data suggests the cancer cells will have been through this stage. This has been debated for some time, but our conclusion is important as it means that screening for Barrett’s is an important approach to controlling Esophageal Cancer.”
Michelle Mitchell, Chief Executive of Cancer Research UK, said, “Today’s insights into the origin of Esophageal Adenocarcinoma could help inform future research efforts into how to diagnose this type of cancer early – which is key for improving patient outcomes.
“This research goes hand in hand with other recent successes in early detection such as Cytosponge, the sponge-on a-string test, which we funded to detect Barrett’s in patients with heartburn symptoms.”
The research was largely funded by the Medical Research Council, Wellcome and Cancer Research UK.
