The study included 124 veterans who completed the test that used a non-endoscopic esophageal balloon device (EsoCheck) to sample tissue that underwent EsoGuard DNA testing. Researchers found fourteen patients with Barrett’s Esophagus (BE) and two patients with Esophageal Adenocarcinoma (EAC), which translated to nearly 13% who were at risk for or had Esophageal Cancer.
Researchers found that the test had 92.9% sensitivity (a highly sensitive test means that there are few false negative results, and thus fewer cases of disease are missed) and 72.2% specificity (specificity of a test is its ability to designate an individual who does not have a disease as negative.) They also determined that the test had a 98.6% negative predictive value (the ratio of subjects truly diagnosed as 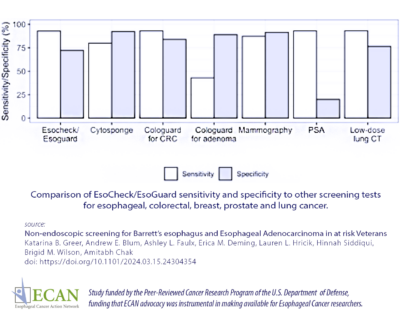 negative to all those who had negative test results).
negative to all those who had negative test results).
 negative to all those who had negative test results).
negative to all those who had negative test results).The authors concluded, “Our data suggest excellent sensitivity and negative predictive value of EC/EG in a screening population of veterans, making this modality a powerful screening tool for BE and EAC.”
The study was funded by the Peer-Reviewed Cancer Research Program of the Department of Defense. ECAN’s advocacy has been instrumental in gaining this funding for scientists researching Esophageal Cancer, adding more than $15 million in funding over the past three years.
You can read the full study here.

