At the 2024 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI) in San Francisco, long-term data from the Phase III KEYNOTE-590 study of first-line pembrolizumab (Keytruda) and chemotherapy for patients with advanced Esophageal Cancer found that the response to this immunotherapy regimen is durable. The landmark trial led to FDA approval of first-line pembrolizumab plus chemotherapy for patients with advanced or metastatic Esophageal Cancer. The trial results show that three times as many patients who received the immunotherapy arm of the study were still alive after five years when compared to those who received chemotherapy alone. No new safety concerns were noted.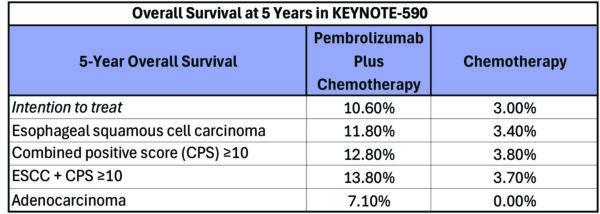
KEYNOTE-590 enrolled 749 patients with locally advanced or metastatic adenocarcinoma (EAC), squamous cell carcinoma of the esophagus (ESCC), or Siewert type 1 gastroesophageal junction adenocarcinoma. They were randomized to receive pembrolizumab plus chemotherapy (5-fluorouracil and cisplatin) or placebo plus chemotherapy.
ECAN’s President and CEO Mindy Mintz Mordecai spoke with Manish A. Shah, MD, Director of the Gastrointestinal Oncology Program at Weill Cornell Medicine during ASCO GI about the significance of the data.
