The U.S. Food and Drug Administration has approved Keytruda (Pembrolizumab) for treatment of advanced Esophageal Squamous Cell Carcinoma (ESCC). It is the first immunotherapy given approval for use in ESCC. Only patients with recurrent, locally advanced, or metastatic ESCC whose Cancer is also positive for PD-L1 qualify for this therapy. Read the FDA announcement here.
How It Works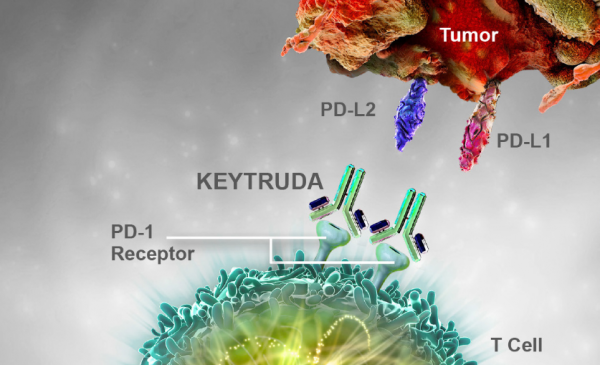
Keytruda is an anti-PD-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. PDL1 is a protein that helps keep immune cells from attacking nonharmful cells in the body. Some cancer cells have high amounts of PDL1. This allows the cancer cells to “trick” the immune system, and avoid being attacked as foreign, harmful substances. Keytruda is designed to inhibit these proteins.
“This is a new, exciting option for patients with esophageal squamous cell cancer,” said Dr. Sarbajit Mukherjee, an assistant professor in the Department of Medicine – GI Medical Oncology at Roswell Park Comprehensive Cancer Center and member of the ECAN Board of Directors.
“Typically, when patients progress after first-line treatment, they are treated with taxane- or irinotecan-based chemotherapy; however, the overall outcomes remain poor. Now, some of our patients can expect to have a treatment option that may help them live longer with possibly less side effects from treatment.”
Improved Survival
The FDA’s approval was based on data from two clinical trials:
- KEYNOTE-181: a multicenter, randomized, open-label, active-controlled trial that enrolled 628 patients with recurrent locally advanced or metastatic esophageal cancer who progressed on or after one prior line of systemic treatment for advanced disease.
- KEYNOTE-180: a multicenter, non-randomized, open-label trial that enrolled 121 patients with locally advanced or metastatic esophageal cancer who progressed on or after at least two prior systemic treatments for advanced disease.
Those studies showed a 22% response rate with 5% of patients experiencing a complete response, compared to a 7% response rate and 1% complete response among patients on chemotherapy. Importantly, Keytruda also appeared to be well tolerated.
Fewer Side Effects
“The really nice thing is that pembrolizumab is very well tolerated so many patients who have difficulty tolerating chemotherapy may be able to handle this medication,” said Dr. A. Craig Lockhart, Professor of Medicine and Chief of the Division of Medical Oncology at the University of Miami – Sylvester Comprehensive Cancer Center who also serves on the ECAN Board of Directors.
Immune-mediated side effects included pneumonitis (inflammation of the lungs), colitis (inflammation of the inner lining of the colon), hepatitis, endocrinopathies (a disease of the endocrine gland), nephritis (a disease that injures the kidney) and renal dysfunction, severe skin reactions, solid organ transplant rejection and complications of allogeneic hematopoietic stem cell transplantation. Find out more about side effects of Keytruda.
Minority of Patients Benefit; More Research is Needed
New treatment options are rare for patients with Esophageal Cancer and more needs to be done to develop new therapies. “We must remember that immune checkpoint inhibitors like Keytruda benefit a minority of patients with Esophageal Cancer,” according to Dr. Muhkerjee. “This emphasizes the need for a better patient selection strategy based on biomarkers as well as conducting further clinical trials with novel agents.”
Dr. Mukherjee recommends patients join clinical trials to help further research that can provide more treatment options for the future. “I would always encourage patients to ask their doctors about potentially participating in clinical trials,” he said. “Esophageal cancer is a deadly disease but scientists and physicians around the globe are working to defeat this cancer. In coming years, I would expect to see (immune checkpoint inhibitors) being approved and used earlier during the treatment course.”
Find out about clinical trials currently recruiting patients with Esophageal Cancer at ECAN’s Clinical Trial Portal.
“Approval of pembrolizumab is a great step forward and most experts expect that we will continue to make progress in the application of immune therapy drugs to the treatment of these cancers,” Dr. Lockhart said. “We will learn to better predict which patients are most likely to respond, we will see new combinations of immune therapies, including vaccines and cellular therapy options are under investigation.”
Also Available for Some Patients with Gastroesophageal Adenocarcinoma
Last year, the FDA granted accelerated approved to Keytruda for patients with previously treated Gastroesophageal Junction Adenocarcinoma – that approval type requires more study. See the FDA announcement here.
